

Wij identificeren genen die de differentiatie van lever- en pancreascellen controleren, en bepalen dan hoe ziektes worden veroorzaakt door dysfunctie van die genen.
De structuur en functie van onze cellen verschillen van één orgaan naar het ander, en het proces waarbij de cellen orgaan-specifieke eigenschappen aanwerven wordt "celdifferentiatie" genoemd. Het laboratorium onderzoekt transgene muizen en humane stalen et heeft daarmee genen ontdekt die celdifferentiatie controleren in lever en pancreas (alvleesklier). In parallel hebben wij gevonden dat dysfunctie van die genen gepaard gaat met ziektes zoals congenitale misvormingen en kanker.

De lever in embryos bestaat essentieel uit hepatoblasten die zich progressief ontwikkelen in hepatocyten of cholangiocyten. Hepatocyten oefenen de metabolische functies van adult lever uit, en cholangiocyten omlijnen de galwegen. Wij hebben genen geïdentificeerd die bepalen hoe hepatoblasten zich ontwikkelen tot hepatocyten of cholangiocyten, en wij bestuderen nu hoe dysfunctie van die genen aanleiding kan geven tot pathologiën. Ten eerste werd er vastgesteld dat hepatocyten in leverkanker kunnen converteren tot kwaadaardige cholangiocyten, en wij determineren in hoeverre dit wordt beïnvloed door differentiatiegenen. Ten tweede, zijn er een aantal metabolische aandoening die in de toekomst door celtherapie zullen kunnen behandeld worden. In dit geval bestaat de celtherapie uit de administratie van in vitro-geproduceerde lever cellen. Het laboratorium kollaboreert met andere onderzoeksteams om in vitro productie te optimizeren, en onze bijdraging hierbij ligt in het omzetten van onze know-how in celdifferentiatie.
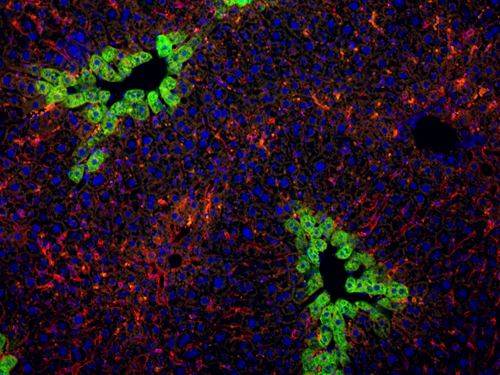
Wij hebben ook genen geïdentificeerd die pancreatische celontwikkeling controleren. Embryonale progenitorcellen in de pancreas geven aanleiding tot adult hormoon-producerende cellen, tot acinaire cellen die spijsverteringsenzymen produceren, en tot canalaire cellen die de pancreatische afvoergangen omlijnen. Zoals in lever kan pancreaskanker geïnitieerd worden door abnormale celdifferentiatie. Wij onderzoeken nu hoe dysfunctie van differentiatiegenen en oncogenen pancreaskanker veroorzaakt et welk pancreatisch celtype (acinair en/of canalair) aan de oorsprong ligt van de tumoren. Een beter begrip van kankerontwikkeling in pancreas en lever moet leiden tot vroeger diagnose en sneller en meer efficiente aanpak van de ziekte bij patiënten.
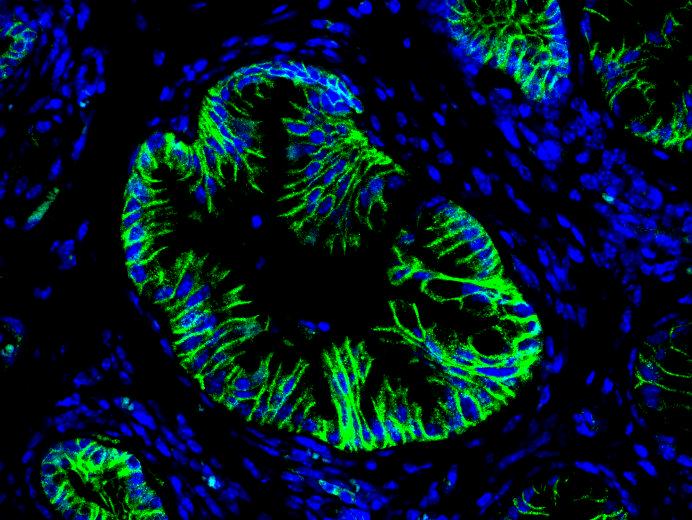
Hepatic cell differentiation
In the embryo, liver progenitor cells (hepatoblasts) derive from the endoderm, the cell layer that delineates the primitive gut. The classical model of liver development predicts that hepatoblasts give rise to hepatocyte precursors, which mature to hepatocytes, and to "ductal plate cells", which generate the cholangiocytes and form bile ducts. Using trangenic mouse models, we have updated the fate map of the hepatic cells in the embryo: the ductal plate cells generate cholangiocytes and bile ducts, but also a subset of hepatocytes, as well as the adult liver stem cells. We now investigate the mechanisms that control acquisition and maintenance of liver cell differentiation in health and disease.
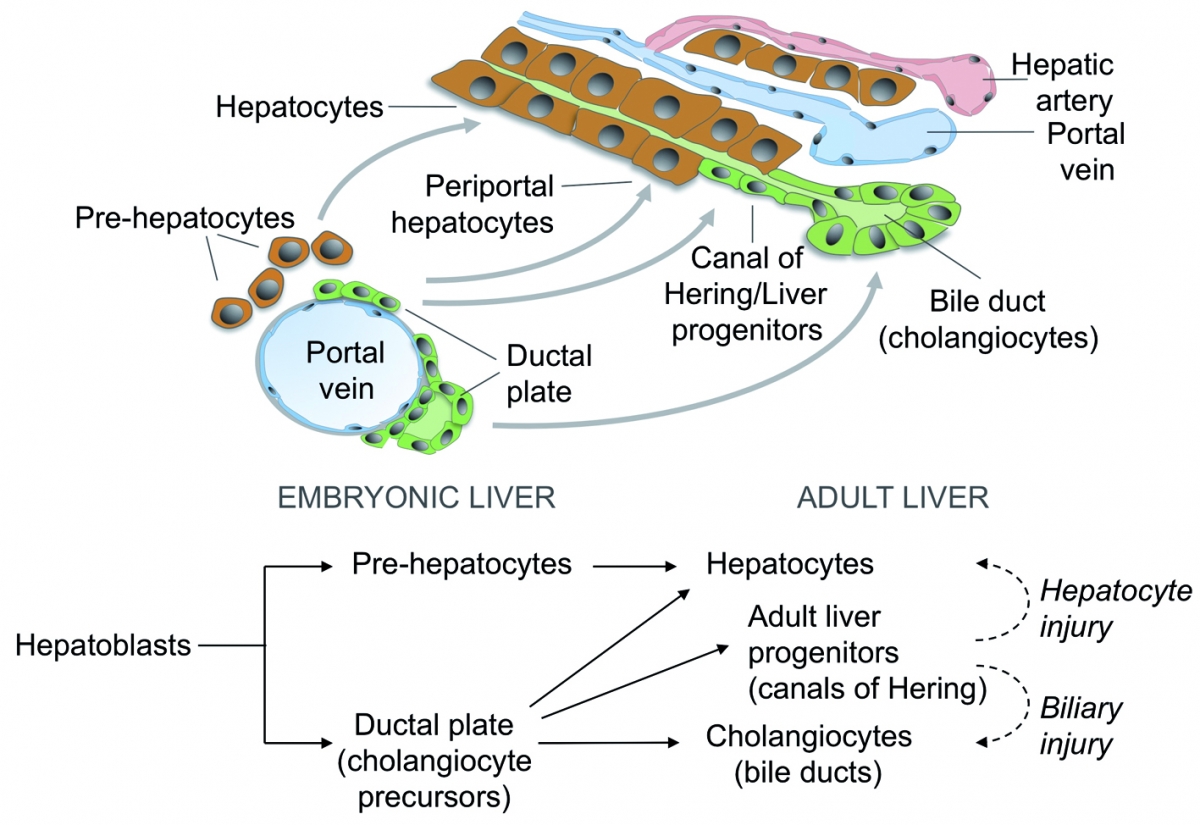
Legend
During development, liver progenitor cells called hepatoblasts give rise to hepatocyte and cholangiocyte precursors. These cells then mature to generate adult hepatocytes forming cords and cholangiocytes delineating bile ducts. Part of the cholangiocyte precursors revert to a hepatocyte phenotype or give rise to adult liver progenitor cells.
Our discovery of the Onecut transcription factors Onecut-1 (OC-1/HNF-6), OC-2 and OC-3, and the subsequent phenotypic characterization of HNF-6 and OC-2 knockout mice led to the identification of transcriptional and signaling networks regulating liver development. We also found that bile duct development proceeds according to a unique process characterized by transient asymmetry. Current efforts are devoted to the characterization of the mechanisms driving three-dimensional morphogenesis of the bile ducts and hepatocyte cords.
In collaboration with clinical centers, we investigate how the knowledge gained from our fundamental studies can be translated in the understanding of the pathophysiology of human biliary diseases, including cholangiocarcinoma. In this context we specifically focus on molecular mechanisms driving formation of precancerous lesions and their progression towards invasive tumor using experimental models that faithfully recapitulate human tumorigenesis.
Pancreatic cell differentiation
Our research on the role of the Onecut transcription factor OC-1/HNF-6 uncovered that this protein is required for development of pancreatic endocrine cells and pancreatic ducts. In the absence of HNF6, endocrine cells fail to develop in the embryo and ducts form cysts. Subsequent research identified transcription-factor microRNA networks in pancreatic cell differentiation; perturbation of these networks causes pancreatic acinar cells to undergo acinar-to-ductal metaplasia, a precursor lesion of pancreatic cancer. Using our extensive expertise in the generation and phenotyping of transgenic mice, we also uncovered that pancreatic duct cells can be at the origin of intraductal papillary mucinous neoplasms, another frequent precursor lesion of pancreatic cancer
Current work is focused on the role of gene regulatory networks, oncogenes and signaling pathways in development of pancreatic adenocarcinoma. Specfic attention is paid to the mode of action of the Kras oncogene and on the role of primary cilia.
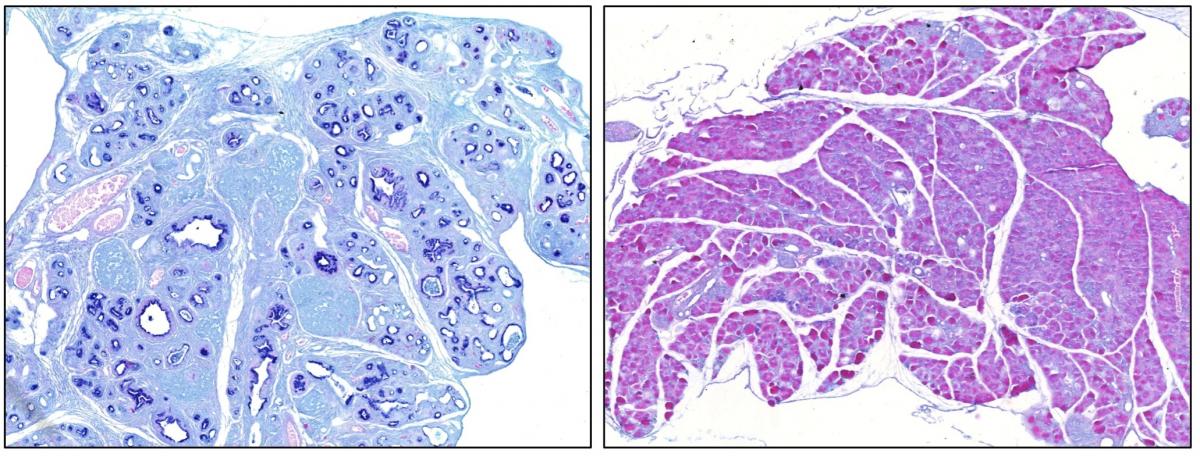
Legend
Alcian Blue staining of section of a pancreas expressing the oncogene Kras (left) and of a pancreas expressing the oncogene Kras in the absence of the transcription factor Sox9 (right). Dark blue staining identifies preneoplastic lesions whereas no such lesions are seen in the absence of Sox9. This result reveals that Sox9 is essential for the initiation of pancreatic ductal adenocarcinoma.
Hepatology. 2021; 74(3):1445-1460.
Cancer Res. 2021; 81(10):2679-2689.
Gut. 2020; 69(4):704-14.
J Hepatol. 2019; 71(2):323-32.
Annu Rev Pathol. 2020; 15:1-22.
J Hepatol. 2018; 68(5):1049-62.
Hepatology. 2018; 67(1):313-27.
Hum Mol Genet. 2016; 25(22):5017-26.
Dev Biol. 2015; 404(2):136-48.
Grimont A, Pinho AV, Cowley MJ, Augereau C, Mawson A, Giry-Laterrière M, Van den Steen G, Waddell N, Pajic M, Sempoux C, Wu J, Grimmond SM, Biankin AV, Lemaigre FP, Rooman I, Jacquemin P
Gut. 2014; 64(11):1790-9.
Prévot PP, Augereau C, Simion A, Van den Steen G, Dauguet N, Lemaigre FP, Jacquemin P.
Gastroenterology. 2013; 145(3):668-78.e3.
Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA.
Gastroenterology. 2012; 143(6):1564-75.e7.
Prévot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J, Bertrand L, Heimberg H, Konieczny SF, Dor Y, Lemaigre FP, Jacquemin P.
Gut. 2012; 61(12):1723-32.
Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP.
Gastroenterology. 2009; 136(7):2325-33.
Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, Rousseau GG, Lemaigre FP.
Genes Dev. 2005; 19(16):1849-54.
Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP.
Development. 2002; 129(8):1819-28.
Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, Rousseau GG, Lemaigre FP.
Mol Cell Biol. 2000; 20(12):4445-54.
Lemaigre FP, Durviaux SM, Truong O, Lannoy VJ, Hsuan JJ, Rousseau GG.
Proc Natl Acad Sci USA. 1996; 93(18):9460-4.

LEVER- EN PANCREASDIFFERENTIATIE IN ONTWIKKELING EN PATHOLOGIE