

Escherichia coli’s envelope is difficult to penetrate and frustrates those who try to find new antibiotics to fight the bacterium. Two of the envelope’s three layers are secured firmly by a lipoprotein, Lpp. By manipulating it, UCLouvain researchers were able to weaken E. coli.
Naturally present in our intestines, Escherichia coli (E. coli) is a great ally of our body and even helps combat certain infections. Why develop antibiotics to fight it? Because certain strains of the bacterium are pathogenic and can cause abdominal cramps and diarrhoea, sometimes with serious complications. Overcoming this requires antibiotics capable of destroying the bacterial envelope, which isn’t easy when faced with a bacterium protected by a three-layered wall.
There are many types of bacteria. E. coli is one of the so-called ‘gram-negative’ bacteria. It thus has three protective layers:
To destroy a pathogenic bacterium, a drug must pass through these layers. ‘Many antibiotics seek to disrupt the assembly of layer 2,’ explains Prof. Jean-François Collet, a bacteria specialist at the de Duve Institute of UCLouvain. ‘We’re not there yet! Because layer 3 can also be very “solid”, especially if it’s solidly anchored to layer 2. This is called bacterial stiffness.’
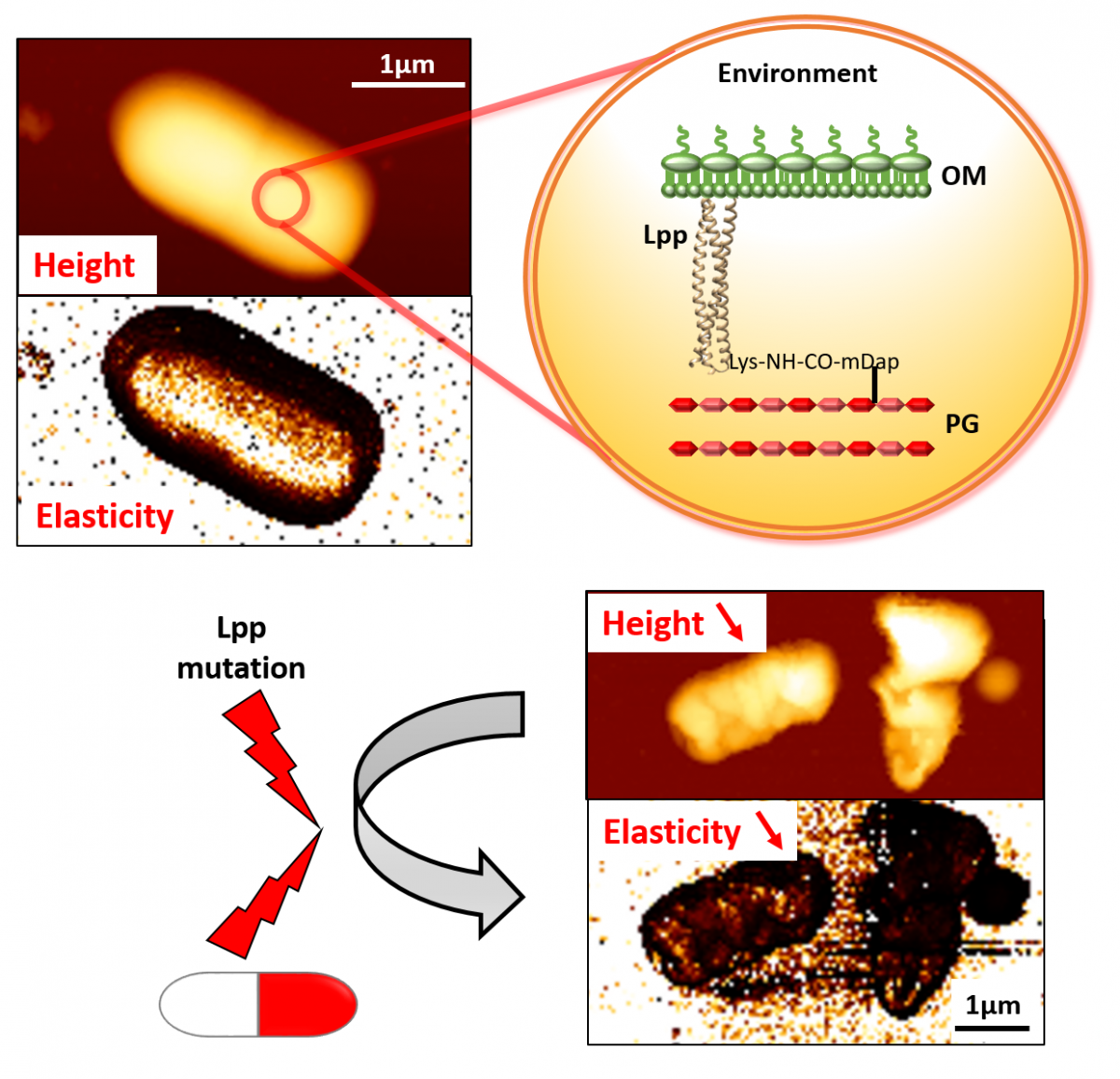
In E. coli, these two layers are attached by numerous copies of the lipoprotein Lpp. Lpp acts as a pillar between the second rampart (layer 2) and the outer enclosure (layer 3).
Prof. Collet and his team have been interested in Lpp for several years. And for good reason. ‘It’s the length of Lpp which determines the space between layers,’ he explains. ‘If we lengthen the Lpp “pillars”, we de facto increase the “ceiling height” and therefore the bacterium’s size. However, modifying Lpp disturbs monitoring layer 3.’
To understand this, we can imagine the bacterium as a fortress. It’s populated with proteins which act as soldiers, masons, or messengers. If the outer perimeter wall (layer 3) is attacked, a messenger is supposed to warn the other proteins, which then intervene to defend or repair the damage inflicted on the bacterium. But if the distance between the perimeter wall and the second rampart (layer 2) has been increased, the message may get lost on the way. The bacterial fortress then becomes deaf and blind to what attacks it.
‘We already knew that manipulating Lpp decreases bacterial stiffness and therefore weakens E. coli's defences,’ Prof. Collet says. ‘But we wanted to know to what extent exactly.’ To do so, the researchers began by genetically modifying E. coli, either by lengthening Lpp or preventing it from attaching to layer 2. Next, Prof. Collet’s team joined that of Prof. Yves Dufrêne, an FNRS research director at the Louvain Institute of Biomolecular Science and Technology (LIBST). ‘Prof. Dufrêne, who also works on bacteria, has extensive atomic force microscope (AFM) expertise. His team has “walked” the AFM’s nanoscale tip on the surface of natural and mutant E. coli bacteria, applying force until they break.’ These experiments confirmed that, yes, manipulating Lpp does indeed reduce bacterial stiffness, regardless of modification (lengthening or detaching) to Lpp. ‘Above all, and for the first time, the AFM has allowed us to quantify and precisely measure the importance of Lpp to the bacterial envelope’s stiffness.’
 |
| The covalent anchorage and molecular length of the lipoprotein Lpp control E. coli mechanical properties and its susceptibility towards antibiotics. |
This study was the subject of an article in the journal Nature Communications. ‘Now that we have these figures, we’d like to find molecules capable of producing the same effects on bacterial stiffness as our genetic manipulations do’, Prof. Collet explains. ‘These are molecules that could disrupt Lpp’s maturation, production and/or action. This would decrease bacterial stiffness and allow antibiotics to more easily enter E. coli’s defences.’ To destroy it, if necessary? That’s the hope and path explored by researchers at UCLouvain.
Article describing this research
Lipoprotein Lpp regulates the mechanical properties of the E. coli cell envelope.
Mathelié-Guinlet M, Asmar AT, Collet JF, Dufrêne YF.
Nat Commun. (2020), 11(1):1789.
Read more
Bacterial Cell Mechanics Beyond Peptidoglycan
Mathelié-Guinlet M, Asmar AT, Collet JF, Dufrêne YF.
Trends Microbiol. (2020), 25:S0966-842X(20)30106-2
Funding
WELBIO (a Wallonia interuniversity research institute); FNRS ‘Excellence of Science’ (EOS) programme; a portion of the European Research Council (ERC) grants obtained by Profs Collet and Dufrêne.