

Converting nutrients from the environment into energy and biomass is a fundamental challenge of life. Unicellular organisms, like bacteria or yeasts, face this challenge in each cell individually. However, when processing nutrients, whose availability is often unreliable, they produce metabolites that function as communication signals to adapt to environmental stress or coordinate growth. An important advantage of multi-cellularity is the ability of cells to metabolically diversify and work together, with metabolites produced in one cell type used as nutrients by another. In complex organisms such as mammals, metabolites are still used for cellular communication, but the extent and importance of these interactions is not well understood.
One example of a tissue in which many different cell types work together is the bone marrow, where daily 200 billion red blood cells, 10 billion white blood cells, and 400 billion platelets are made. The correct functioning of this process relies not only on blood stem cells, but also on their interactions with other cell types in the bone marrow microenvironment such as bone cells, blood vessel cells, immune cells and connective tissue cells, collectively referred to as the bone marrow niche. The bone marrow niche is also involved in the development and progression of blood malignancies such as leukemia, in which blood stem cells start to grow uncontrollably and no longer form normal blood cells.
Our laboratory explores how metabolic communication between different cells in the bone marrow regulates blood stem cell function, blood cell production and leukemia growth. We use state-of-the-art metabolic analysis techniques in combination with cell and animal models to investigate how metabolites produced by one cell type influence the behavior of the other cells in its environment. The goal of our research is to identify niche-derived metabolic signals important for the maintenance of blood stem cells and the production of red blood cells, white blood cells and platelets. This information can then be used to improve bone marrow transplantation or help in the treatment of blood diseases. By comparing normal and leukemic blood cell production, we further aim to identify changes in metabolic communication systems that can be targeted to reduce the growth of leukemia cells or improve the efficacy of current treatment methods such as chemotherapy.
Blood cell production in adults occurs in the bone marrow, where hematopoietic stem and progenitor cells are supported by niche cells that provide essential factors for hematopoietic cell maintenance, proliferation and differentiation. The bone marrow niche is complex, consisting of mesenchymal stromal cells, osteolineage cells, adipocytes, endothelial cells, perivascular cells, nerve cells and megakaryocytes, which perform both distinct and overlapping functions in hematopoietic support. The bone marrow niche is also involved in the development and progression of hematopoietic malignancies such as leukemia, where competition with healthy stem and progenitor cells and niche remodeling occurs.
Research into the bone marrow niche has focused primarily on identifying cellular sources of essential hematopoietic cytokines such as CXCL12, stem cell factor and thrombopoietin. However, these ligand-receptor signaling pathways are layered upon more evolutionary ancient metabolic communication mechanisms that are poorly understood. Our goal is to unravel the importance of metabolic crosstalk between different cell types in the bone marrow microenvironment for normal and malignant hematopoiesis. Detailed characterization of these metabolic interactions, and how they are altered in the setting of disease, can provide new therapeutic opportunities for hematological disorders, regenerative medicine and cancer.
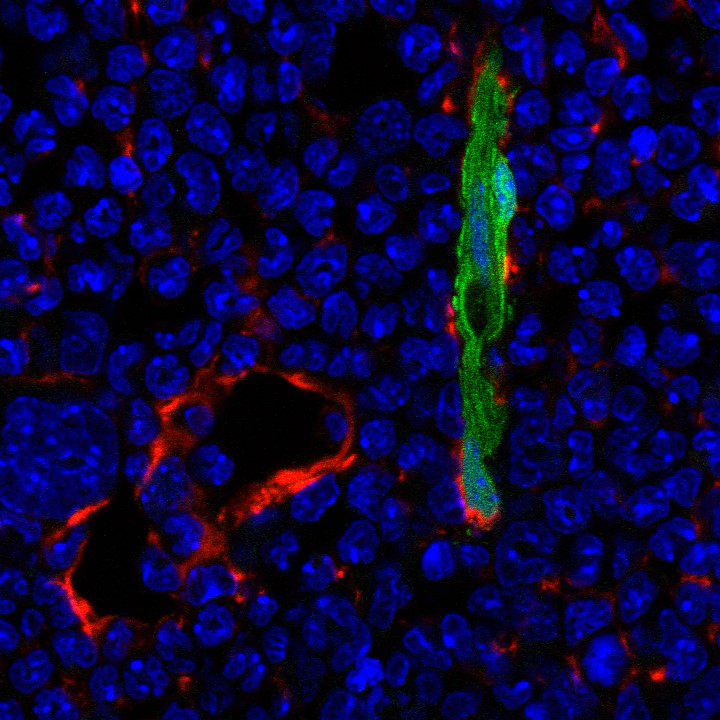
Immunohistochemical staining showing Nestin-expressing periarterial cells (GFP, green) and Leptin Receptor-positive stromal cells (Cy3, red) in the mouse bone marrow microenvironment. Cell nuclei are visualized using Hoechst (blue).
PROJECTS:
Stromal metabolites in hematopoiesis and leukemia
Aspartate is a non-essential amino acid with diverse metabolic functions in the cell. In our previous research, we showed how aspartate produced by bone marrow stromal cells is used by acute myeloid leukemia cells to fuel nucleotide production (van Gastel et al. Cell Metabolism 2020). In this project, we aim to understand the importance of extracellular aspartate for normal hematopoietic and leukemia cell growth, and investigate the identity of the aspartate-secreting cells in the bone marrow microenvironment. We will also explore additional metabolic exchanges between stromal and hematopoietic cells.
Microenvironmental contributions to chemotherapy resistance in leukemia
Chemoresistance is the difference between remission and cure in cancer patients and is particularly evident in acute myeloid leukemia, where complete remissions are common, but few are cured. Our goal is to understand how communication with other cells in the bone marrow microenvironment helps rare leukemia cells persist during chemotherapy treatment, and how these interactions can be therapeutically targeted to improve patient survival.
Fatty acid sensing and metabolism in leukemia
Fatty acids are important dietary fuel sources and essential structural components of cells. How cells sense the availability of fatty acids in their direct environment remains poorly understood. We have shown that FoxO transcription factors play a role in the response to fatty acid deprivation (van Gastel et al. Nature 2020). Current efforts are focused on further unraveling the molecular structure of this mechanism and its importance for cell growth and survival. As part of this research, we also aim to understand how fatty acid sensing and other aspects of fatty acid metabolism are dysregulated in the setting of leukemia.
References
van Gastel N., Spinelli J.B., Sharda A., Schajnovitz A., Baryawno N., Rhee C., et al. Induction of a timed metabolic collapse to overcome cancer chemoresistance. Cell Metabolism 2020, 32 (3):391-403.
van Gastel N., Stegen S., Eelen G., Schoors S., Carlier A., Daniëls V.W., et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 2020, 579 (7797):111-117.
