APPLICATIONS
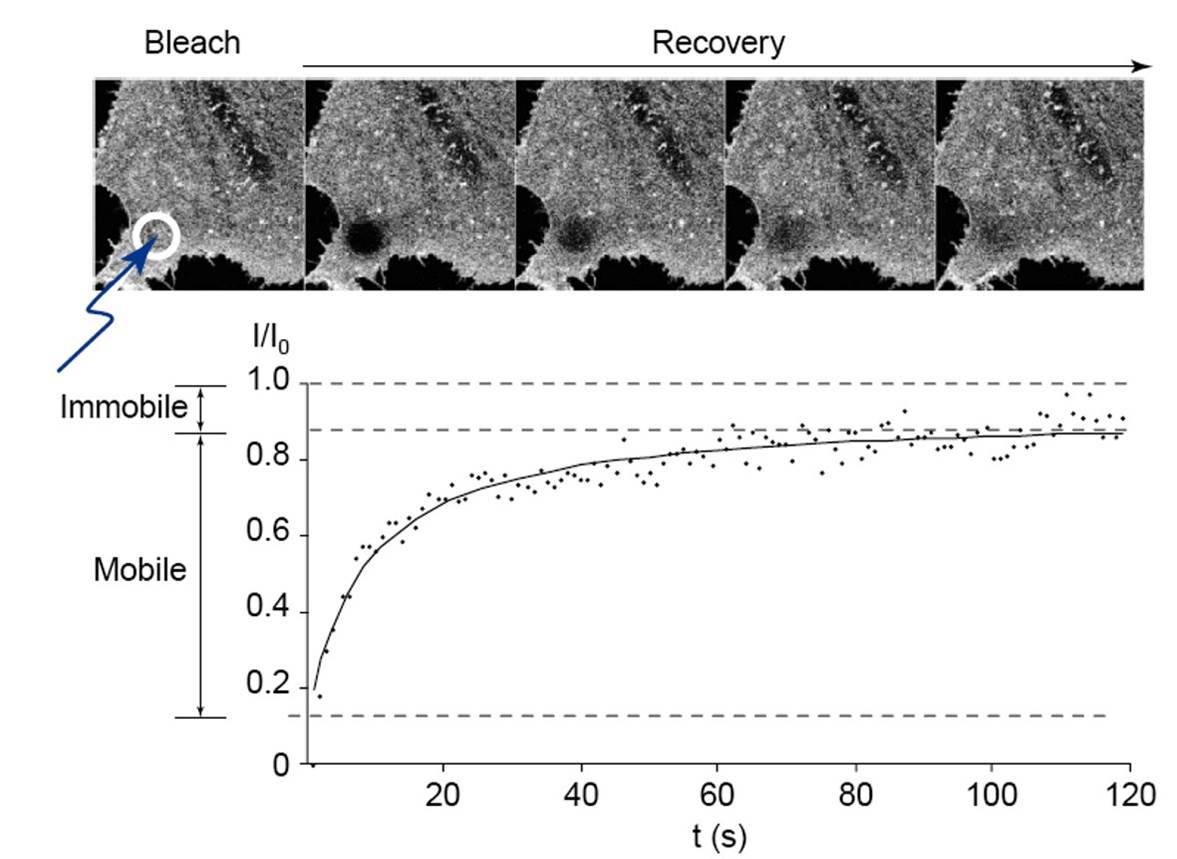
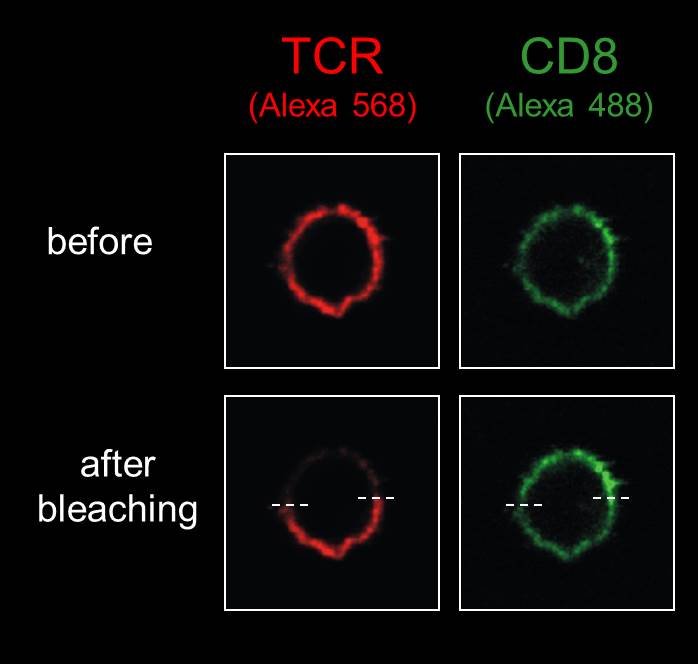
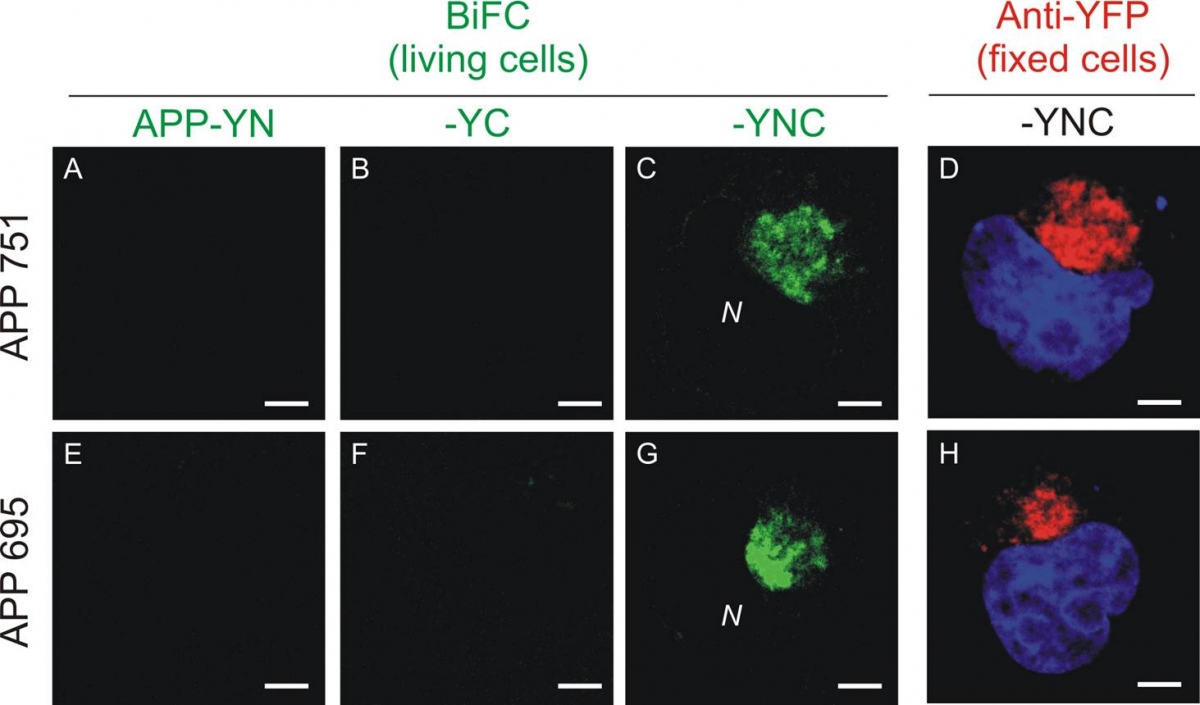
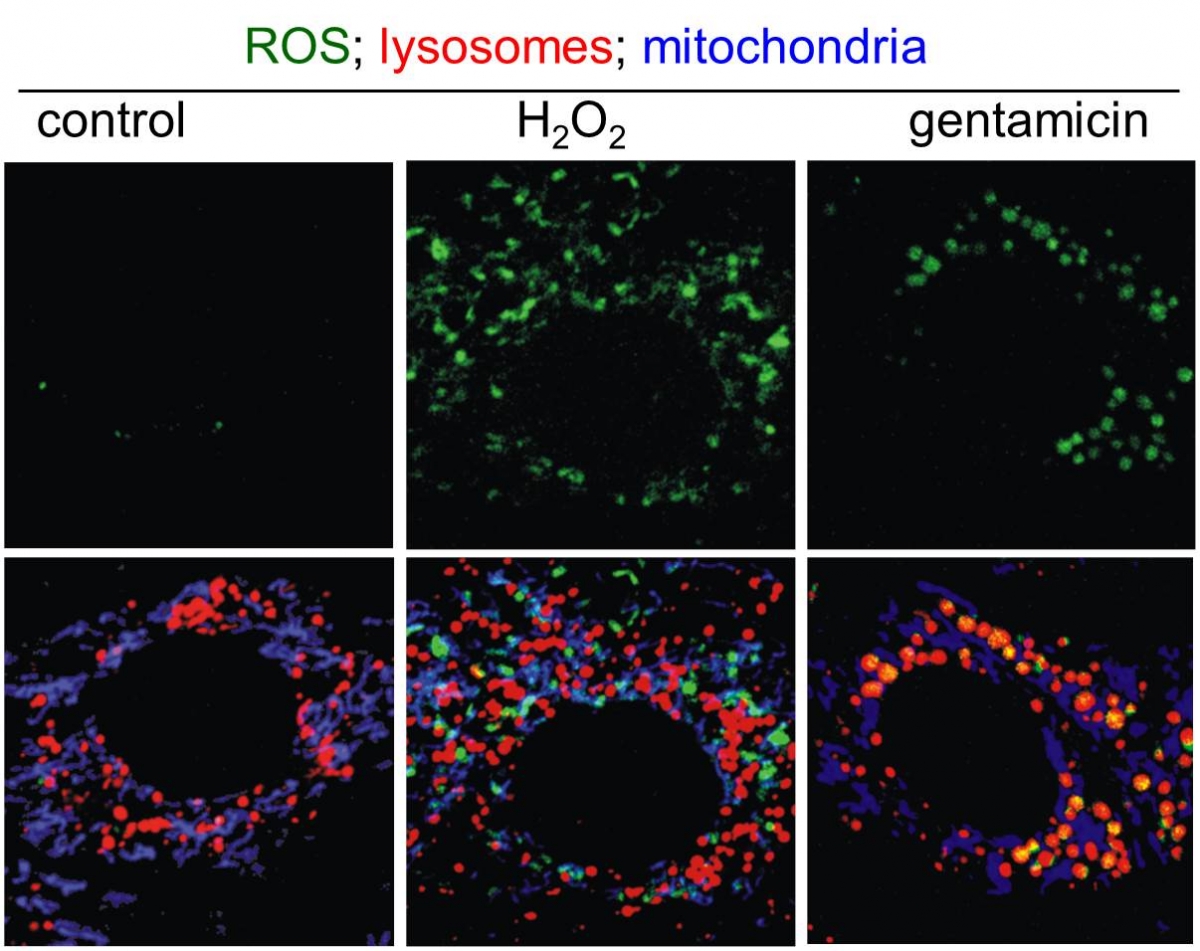
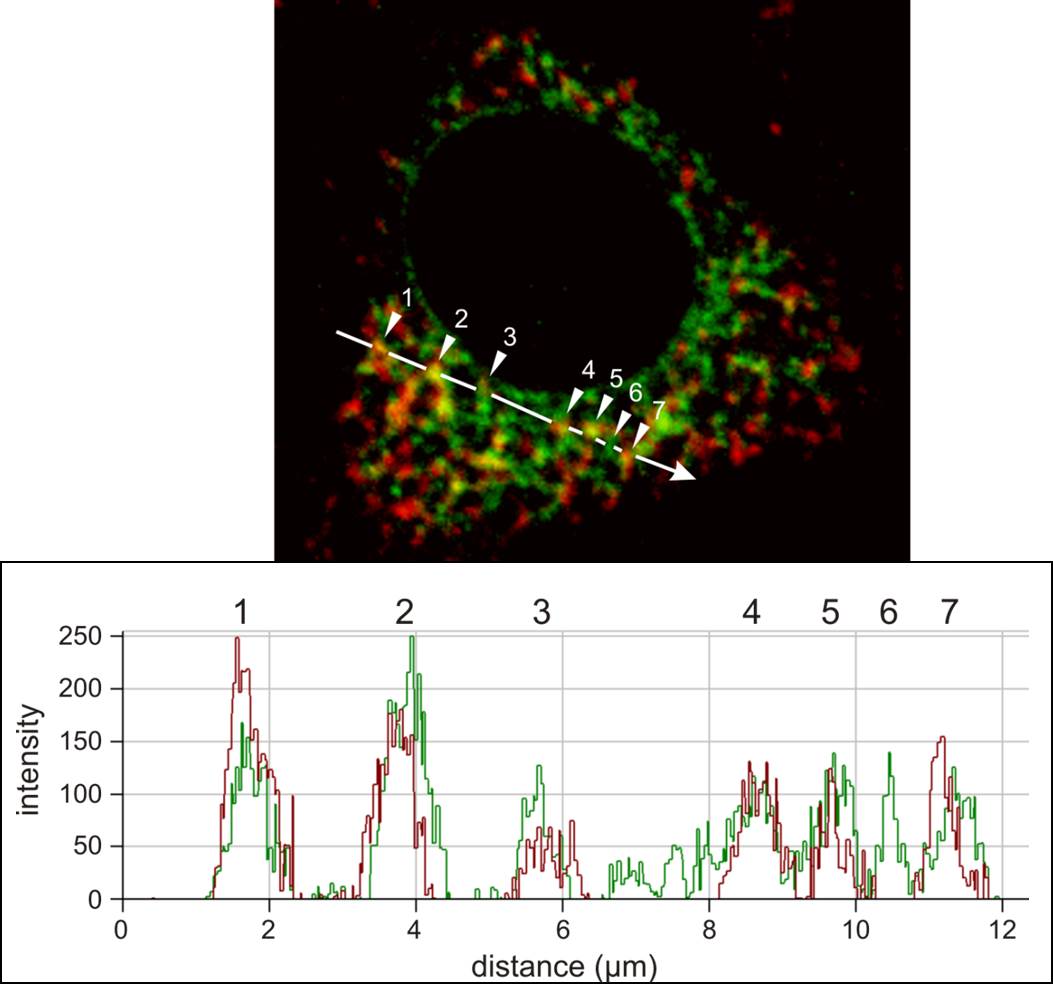
We are happy to share expertise in scientific projects addressing defined questions which can benefit from: (i) electron microscopy (scanning and transmission), including ultrastructural cytochemistry; (ii) high-resolution and high-throughput confocal microscopy, for live-cell imaging and immunolabelling; (iii) multiphoton microscopy to image tissues and organs for several hours without damage; and (iv) advanced applications by confocal microscopy, including dynamics of molecular movement by Fluorescence Recovery After Photobleaching (FRAP), protein dimerization by Bimolecular Fluorescence Complementation (BiFC), in situ detection of free radicals (ROS) at distinct subcellular compartments, and protein:protein interactions by FRET, etc. In addition, we propose advanced images analyses such as deconvolution, 3-D reconstruction, morphometry, objective co-localization, etc.
EXPERTISES
1. Basic training and supervision of routine imaging.2. Advanced training destined for independent, registered platform users.
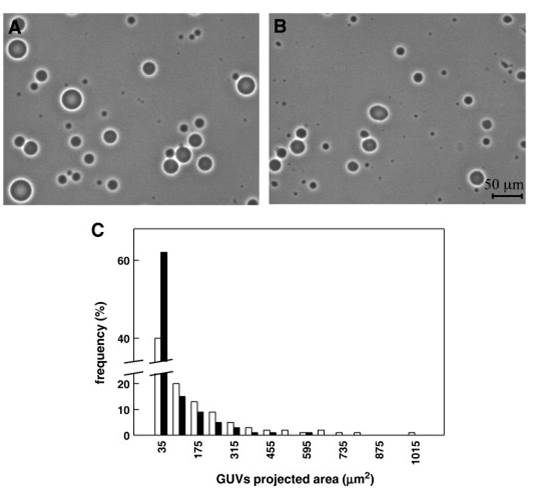
3. Sophisticated methods for preparation of biological samples (cells, tissues and organs).
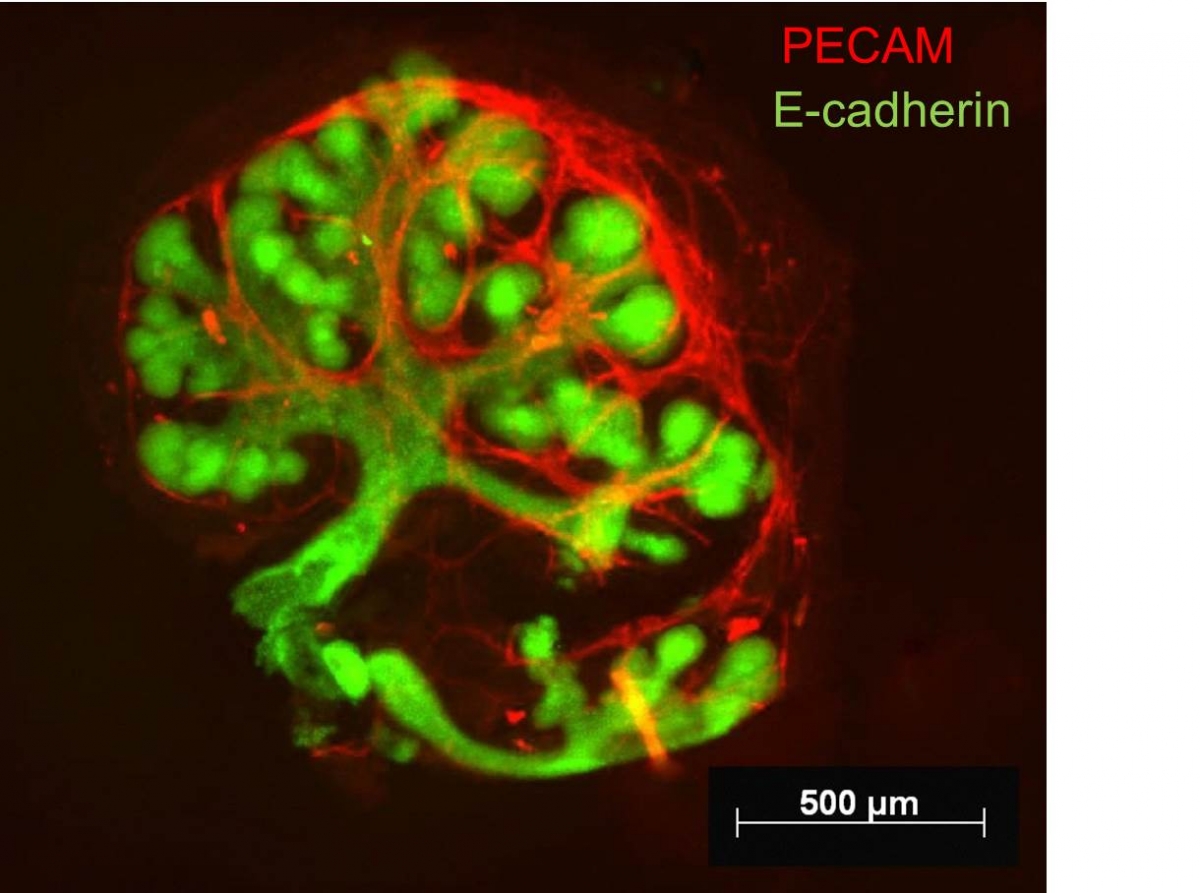
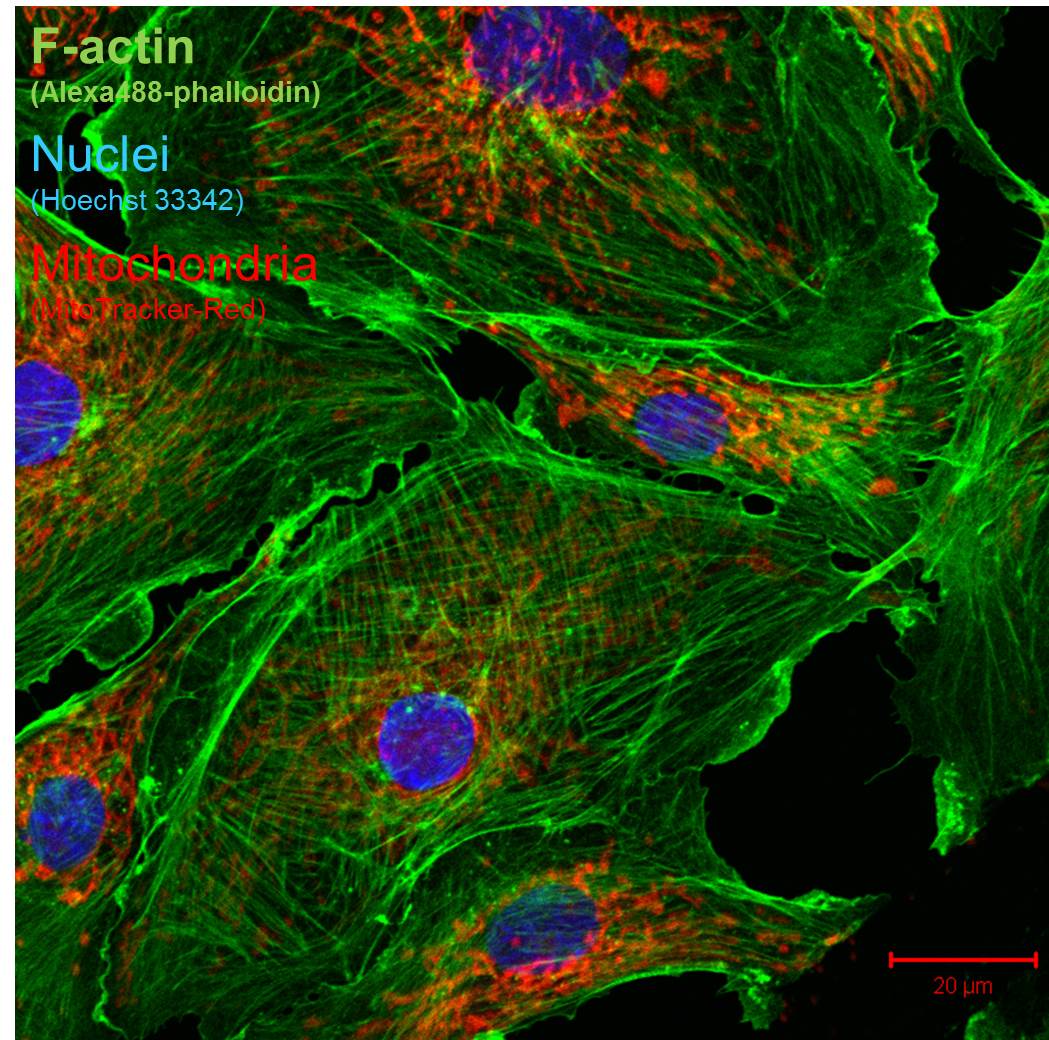
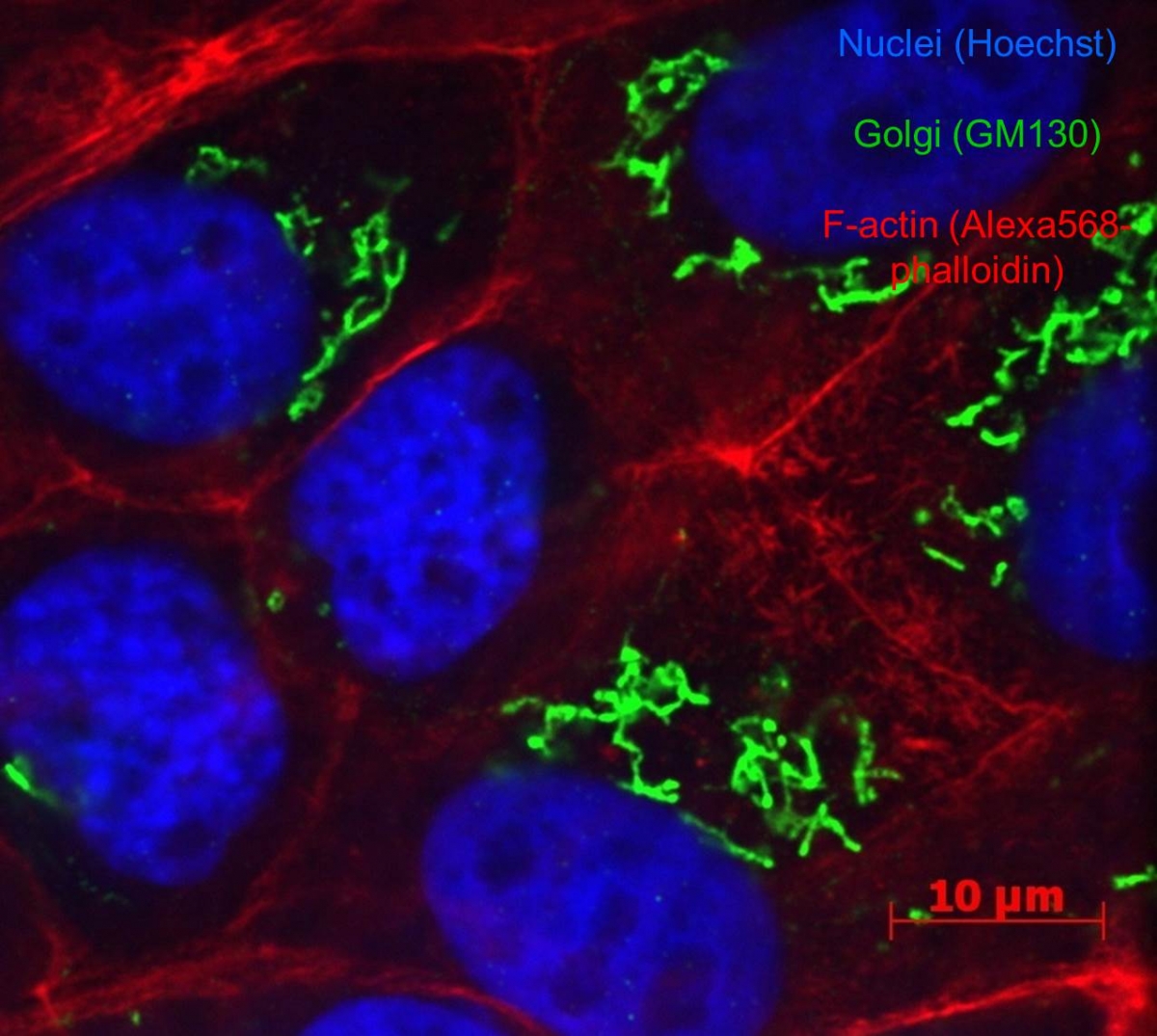
4. Fluorescence imaging on fixed cells and tissues.Salivary glands explants (unpublished data).
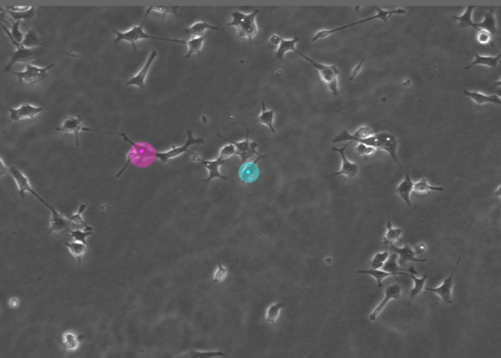
5. Fluorescence imaging on living cells: cell migration.
|
11. Advanced applications by confocal microscopy:
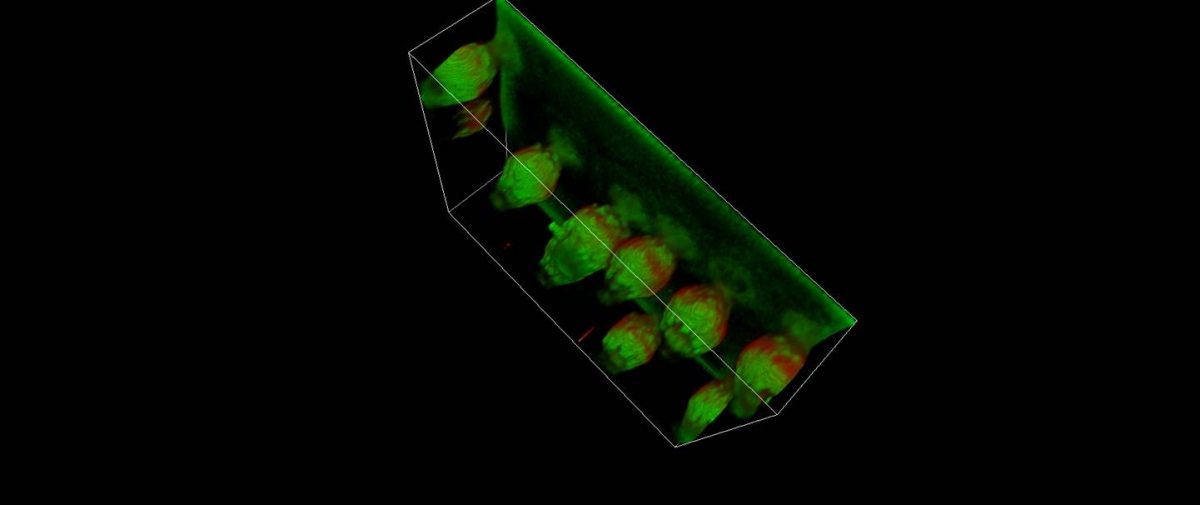
12. Advanced off-line image analysis:
|